MacroFLUX
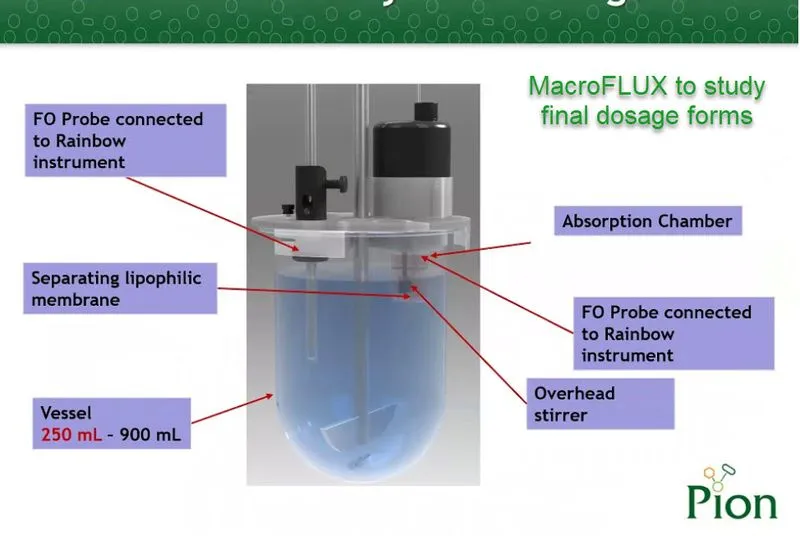
The need to test finished drug products for their absorption potential in order to predict pharmacokinetic performance has been a topic of great interest to the drug development community. In many cases, dissolution experiments alone cannot correctly predict the in vivo response to drug products due to the complicated interplay of solubility and permeability in complex media. Pion’s MacroFLUX device extends the utility of in situ concentration monitoring to improved assessment of absorption potential and more realistic IVIVC modeling.
Introducing a stirred absorption chamber into the traditional USP I and II apparatus allows this type of testing to be done in vitro through the use of the MacroFLUX dissolution system. The USP vessel is the donor compartment, allowing for the volumes needed to test finished dosage forms under sink conditions. Using in situ fiber optic UV detection in both the donor and receiver provides the required data density for accurate assessment of transmembrane FLUX.
Contact us to learn more.

