BioFLUX
BioFlux™ system can be combined with the in situ Fiber Optic UV System to study absorption potential of drugs final dosages.







BioFlux™ system can be combined with the in situ Fiber Optic UV System to study absorption potential of drugs final dosages.
Used for conducting simultaneous dissolution-absorption studies.
Assess the complex of interplay between solubility, permeability and dissolution rate in-vitro.
Evaluate whether certain formulation changes will affect the bio-performance of a certain drug product.
BioFlux™ system can be combined with the in situ Fiber Optic UV System to study absorption potential of drugs final dosages.
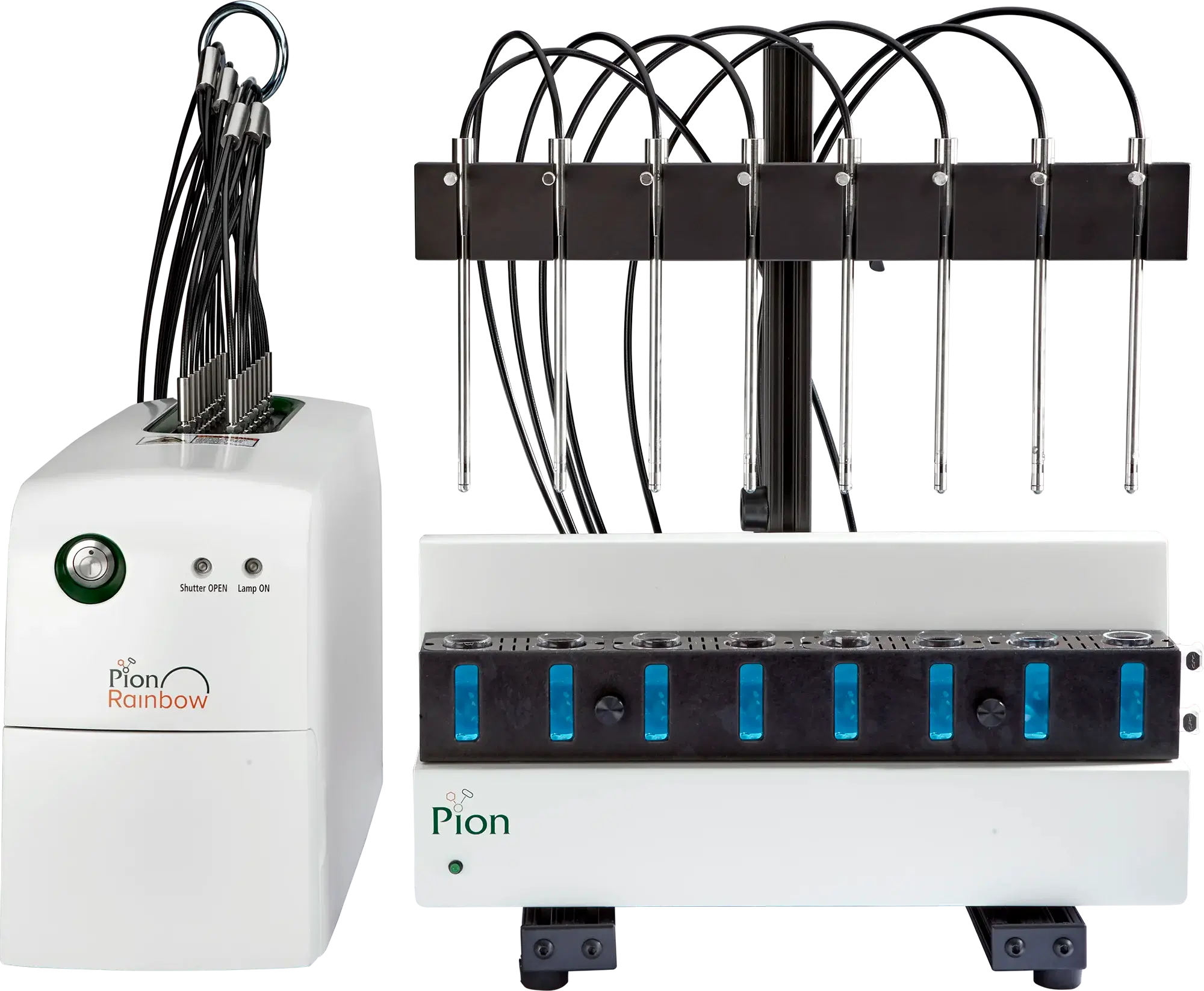
The In-Situ UV Probe-Based Dissolution System
The BioFLUX™ system consists of an absorption (receiver) chamber integrated with a lipophilic membrane, overhead stirrer and fiber optic (FO) UV probe that is inserted in to a modified cover of a miniaturized 500 mL vessel of USP II dissolution apparatus.
The phospholipid-covered artificial permeation membrane separates the dissolution (donor) compartment from the receiver compartment, containing Pion Acceptor Sink Buffer at pH 7.4. The dissolution compartment typically holds 200 – 250 mL of compendial or biorelevant dissolution buffer. The modified vessel cover allows fiber-optic UV probes to be positioned in both the donor and receiver compartments to monitor real-time concentration kinetic in both chambers. Concentration monitoring is enabled by connecting the fiber optic UV probes to the Rainbow Dynamic Dissolution Monitor® instrument.
.avif)



Improve Process and Decision Making with Better In Vitro Insights
The BioFLUX™ in conjunction with Rainbow R6
- Provided valuable information about permeability-solubility interplay of poorly soluble drugs during formulation development;
- Allows to perform experiments at biorelevant volumes (200-250mL) for late stage formulation development or bioequivalence studies;
- Designed to work with USP II apparatuses.