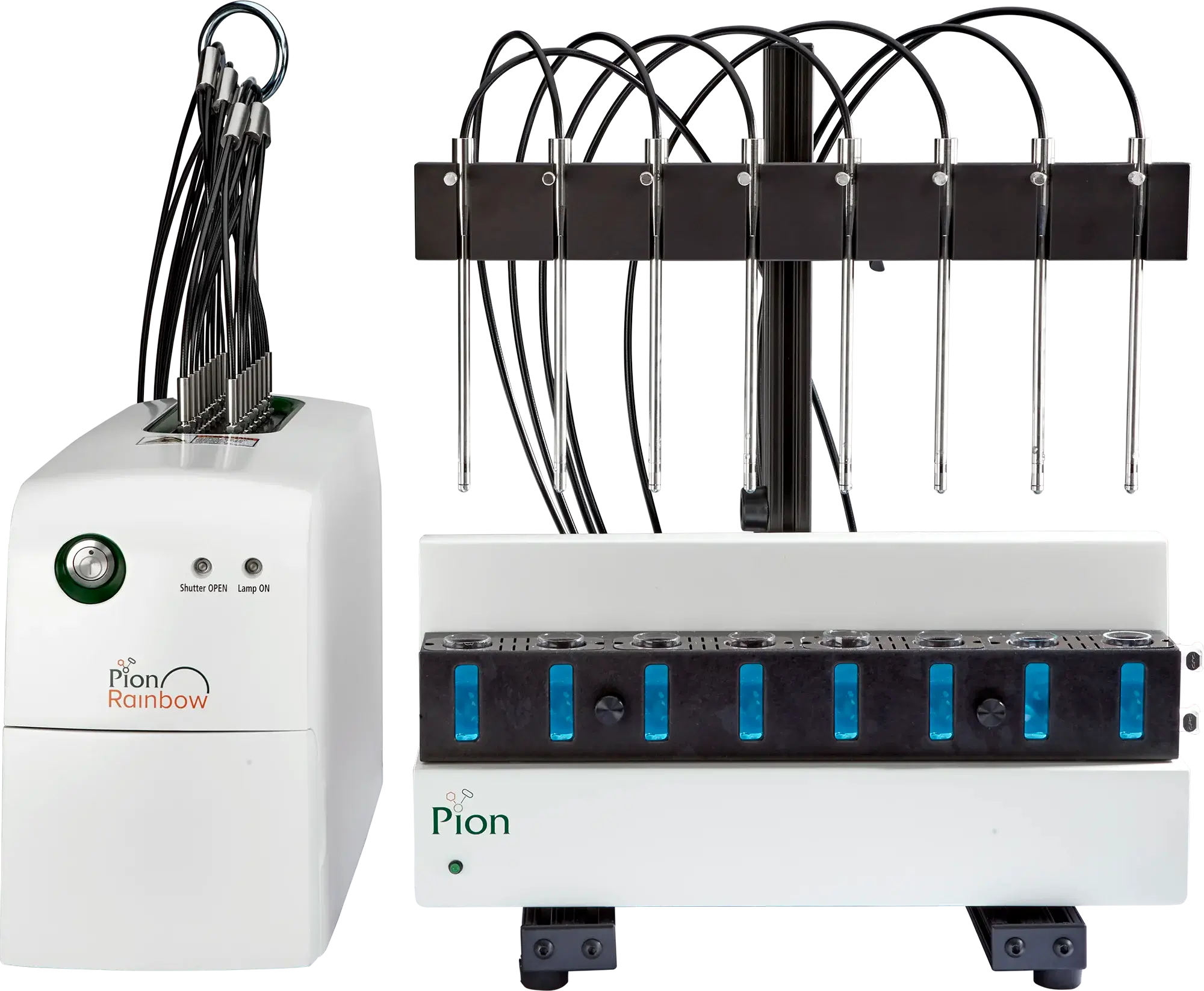
Rainbow R6 for QC and R&D
The in situ fiber optic UV-Vis spectrometer enables real-time concentration monitoring for dissolution, absorption, and solubility studies.







Configurations
User Applications
Coupled with dissolution bath
- Small volume dissolution testing
- Solubility measurement
- Dissolution rate and IDR measurements
- Supersaturation-precipitation studies
- Salt and polymorph selection
Coupled with dissolution-permeation (flux) system:
- Absorption prediction
- Prediction of the rate limiting step of absorption
- Bioequivalence prediction
- Formulation excipient effect screening
- Formulation strategy selection
Coupled with Subcutaneous Injection site simulator (Scissor):
- Biosimilar characterization
- Injectable formulation rank ordering
- De-risking formulation issues upon injection



Software for the Rainbow R6
AuPRO software for Rainbow for data collection and refinement in research and development:
A powerful analytical software designed specifically for the needs of early-stage compound screening, and during pre-formulation and formulation development.
DissoPRO software for Rainbow for method development and quality control (QC)
A secure, traceable software package designed to address the needs of dissolution scientists and quality groups in highly regulated environments.
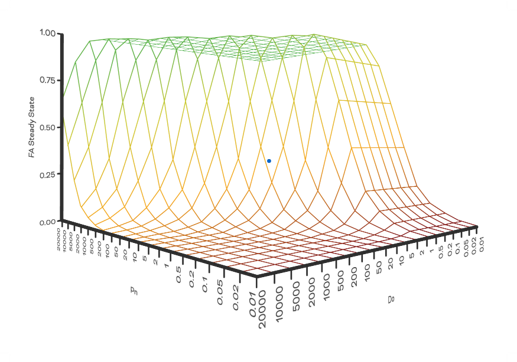
Pion’s Predictor Software applies the mechanisms of the Gastrointestinal Unified Theoretical (GUT) framework to convert in vitro flux data to predictions of in vivo oral absorption and percent fraction drug absorbed (Fa%). When you apply Predictor software to flux data collected by Pion’s Rainbow Dynamic Dissolution Monitoring system, the Predictor data can help you understand the interplay between dose (Do), dissolution (Dn,) and permeability (Pn) to help you understand where to target formulation improvements.
Predictor assigns an estimation of the rate-limiting step to absorption of the API based on the Do, Dn and Pn numbers calculated from the Fa% and defines whether the compound is subject to a permeability, dissolution, or solubility-permeability limitation.
Additionally, Predictor is able to classify the drug according to the Biopharmaceutics Classification System (BCS) based on the Do and Pn numbers determined from the Fa% result. Formulation scientists can then decide on a strategy for improving oral absorption outcome by overcoming the significant rate-limiting steps for their drug.
Additional Benefits
- 5 different advanced mathematical approaches to deal with turbidity or other disturbances in the spectrum, making in situ monitoring work
- Its Green. No filters, HPLC vials, columns or eluents. Everything is done in your experiment vessel
- Direct measurements in complex or biorelevant media, even with media conversion is possible in small, biorelevant or USP volume
- No separation is needed. Multiple mathematical ways to measure 2-3(-4) absorbing components in the vessel, all in real time