MacroFLUX
In-vitro assessment of dissolution-absorption processes from full dosage forms.







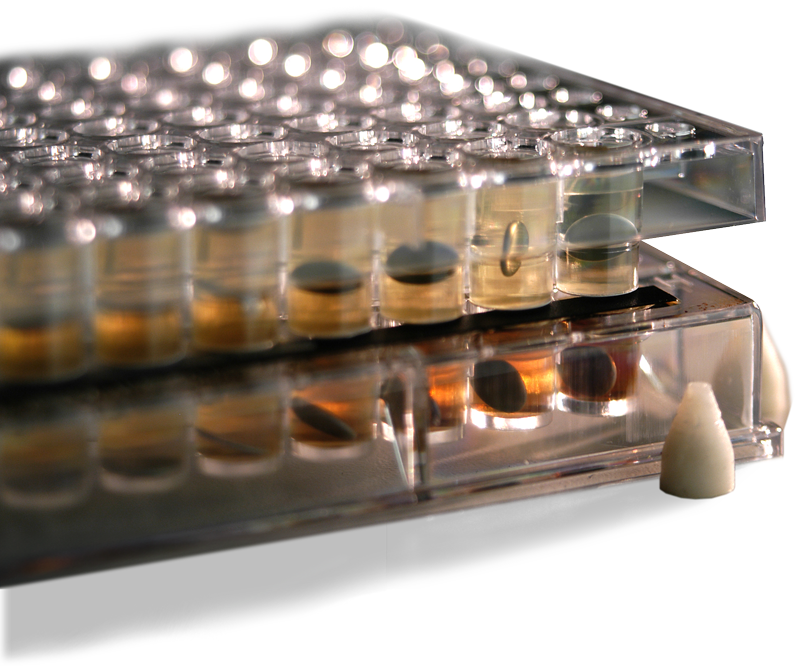

PAMPA
Pion’s PAMPA is an in vitro, high throughput screening assay highly correlated to biological membrane permeability.

LOREM IPSUM
Screening excipients, solvents, and APIs to rank order formulations and drug candidates on the basis of permeability.

LOREM IPSUM
Each PAMPA well uses artificial lipid membranes that mimic drug uptake in the gastrointestinal tract (GIT) or the blood-brain barrier(BBB) to provide robust, reliable, bio relevant results.
In-vitro assessment of dissolution-absorption processes from full dosage forms.
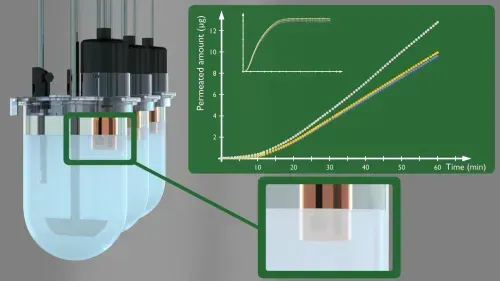
Combine Dissolution and Absorption measurements into a single assay.
A stirred absorption chamber into familiar dissolution apparatus
Extend the utility of in situ UV concentration monitoring to provide an accurate assessment of transmembrane FLUX from whole dosage forms
Real-time dissolution and absorption concentration monitoring
The filter-supported artificial permeation membrane separates the dissolution (donor) compartment from the receiver compartment, containing pH 7.4 Pion Acceptor Sink Buffer.
The dissolution compartment typically holds 900 mL of compendial or biorelevant dissolution buffer. The modified vessel cover allows fiber-optic UV probes to be positioned in both the donor and receiver compartments allowing real-time dissolution and absorption concentration monitoring in both chambers. Concentration monitoring is enabled by connecting the fiber optic UV probes from the Rainbow Dynamic Dissolution Monitor® instrument.

THE PION PAMPA ADVANTAGE
Lorem ipsum dolor sit amet, consectetur adipiscing elit.
In Vivo Correlation
Gut-Box controls stirring speed to match the hydrodynamics of the intestinal tract or the blood brain barrier. The adjustable stirring allows you to mimic the ABL (aqueous boundary layer), allowing for higher accuracy and reducing assay time.[1].
High Throughput
The STIRWELL plates in Pions’ PAMPA sandwich kits contain a pre-loaded stir bar in each well, providing dynamic stirring for each assay, allowing for high-throughput screening of multiple compounds simultaneously.
Easy Data Interpretation
PAMPA Explorer software for easy data
interpretation. SW includes a plate-reader
data wizard, results visualization, and reporting. Data output is the complete mass balance including membrane retention.
Reproducible Results
While orbital shakers can lead to edge
effects in the assay plates, GutBox provides consistent agitation across the whole plate, allowing for more reproducible data.
Complete Solution
The complete PAMPA system includes our 96well STIRWELL plates, the Gut Box™ stirring system and PAMPA Explorer™ software.

Pion’s PAMPA is an in vitro, high throughput screening assay highly correlated to biological membrane permeability
Screening excipients, solvents, and APIs to rank order formulations and drug candidates on the basis of permeability.
Each PAMPA well uses artificial lipid membranes that mimic drug uptake in the gastrointestinal tract (GIT) or the blood-brain barrier(BBB) to provide robust, reliable, bio relevant results.

MacroFLUX can be used:
- To conduct simultaneous dissolution-absorption studies
- During formulation development for optimizing product performance and to evaluate whether certain formulation changes will affect the bio-performance of a certain drug product
- During life-cycle management and product extensions to demonstrate bioequivalence
- To compare brand and generic formulations to demonstrate bioequivalence
- To compare test to reference formulations
- To ensure continuity of product quality (batch to batch consistency) and performance of the manufacturing process (in order to differentiate bioequivalent batches from non-bioequivalent batches within a range that guarantees comparable biopharmaceutical performance in vivo).








