Quality Control
DissoSpec QC Dissolution Monitoring System
Dissolution data – now live!







Integrated, in situ UV-Vis dissolution testing system for efficient and compliant method development and Quality Control (QC) dissolution analysis.
No items found.
In Situ UV Probe-Based Dissolution System
Dosage to Data, Simplified
- Real-time data analysis with fiber optic probes
- High resolution dissolution profiles
- Develop methods for transfer to QC
- Simplified data collection and interpretation


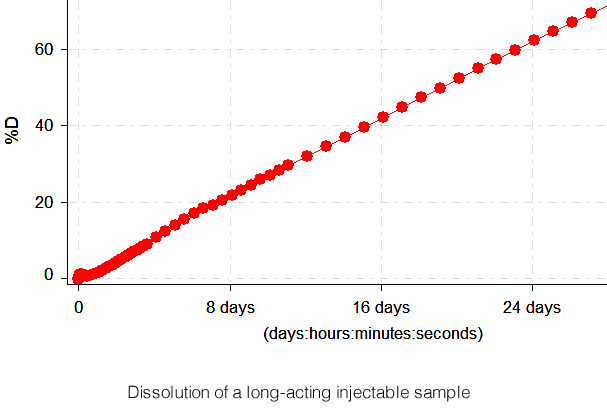
DissoSpec is engineered to meet USP 1 / 2 / 5 / 6 dissolution methods, ensuring compliance while reducing operational costs.
Advanced UV-Vis Technology:
- In situ analysis ensuring stable and reliable data collection.
- In situ data acquisition: Acquire full spectral readings as fast as every 5 seconds.
- 2nd derivative spectroscopy: Quantify and resolve dual-component dosages accurately, without filtration or physical separation of sample components.
Long-Acting Injectable (LAI) Adapters
- Simplify sample introduction, improve reproducibility and discrimination, and increase resolution with LAI devices from Pion.
- Enables dissolution testing of long-acting injectables and other extended-release dosage formulations
Long-Acting Injectable Adapter, USP 2 Dissolution Apparatus
Volume range from 5 μL – 100 μL


Long-Acting Injectable Adapters, USP 4 Flow-through cells
Samples 50 μL and 1 mL in volume


* Can be used with Pion’s DissoSpec QC Dissolution Monitoring System.
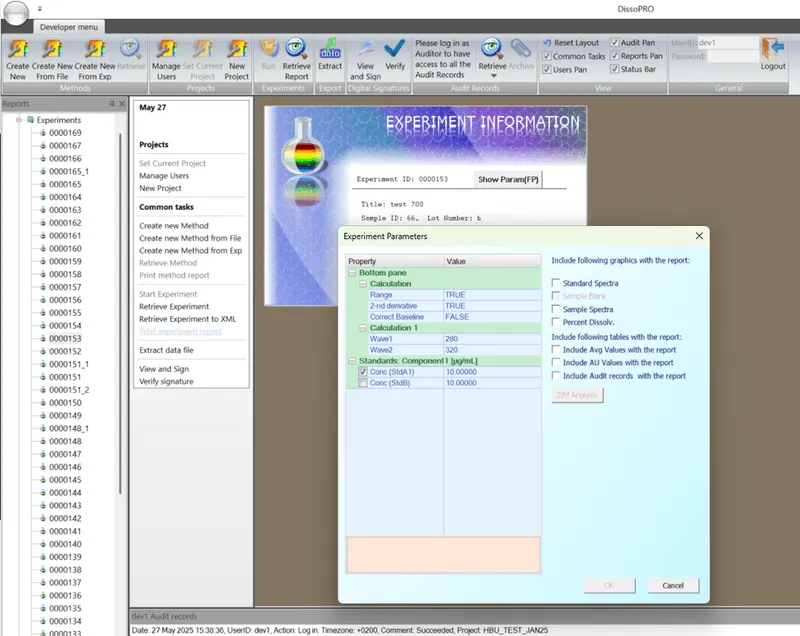
DissoPRO™ Software with 21 CFR Part 11 Compliance
Enhanced Data Security:
- Full access controls, audit trails, electronic signatures, and ALCOA++ compliance.
User-Friendly Interface:
- Simplified dissolution run protocols with secure login roles that meet current GMP/GLP standards.
Automatic Report Generation:
- Receive a single, consolidated report upon dissolution completion, covering standard system suitability test, dissolution parameters and data analysis.



