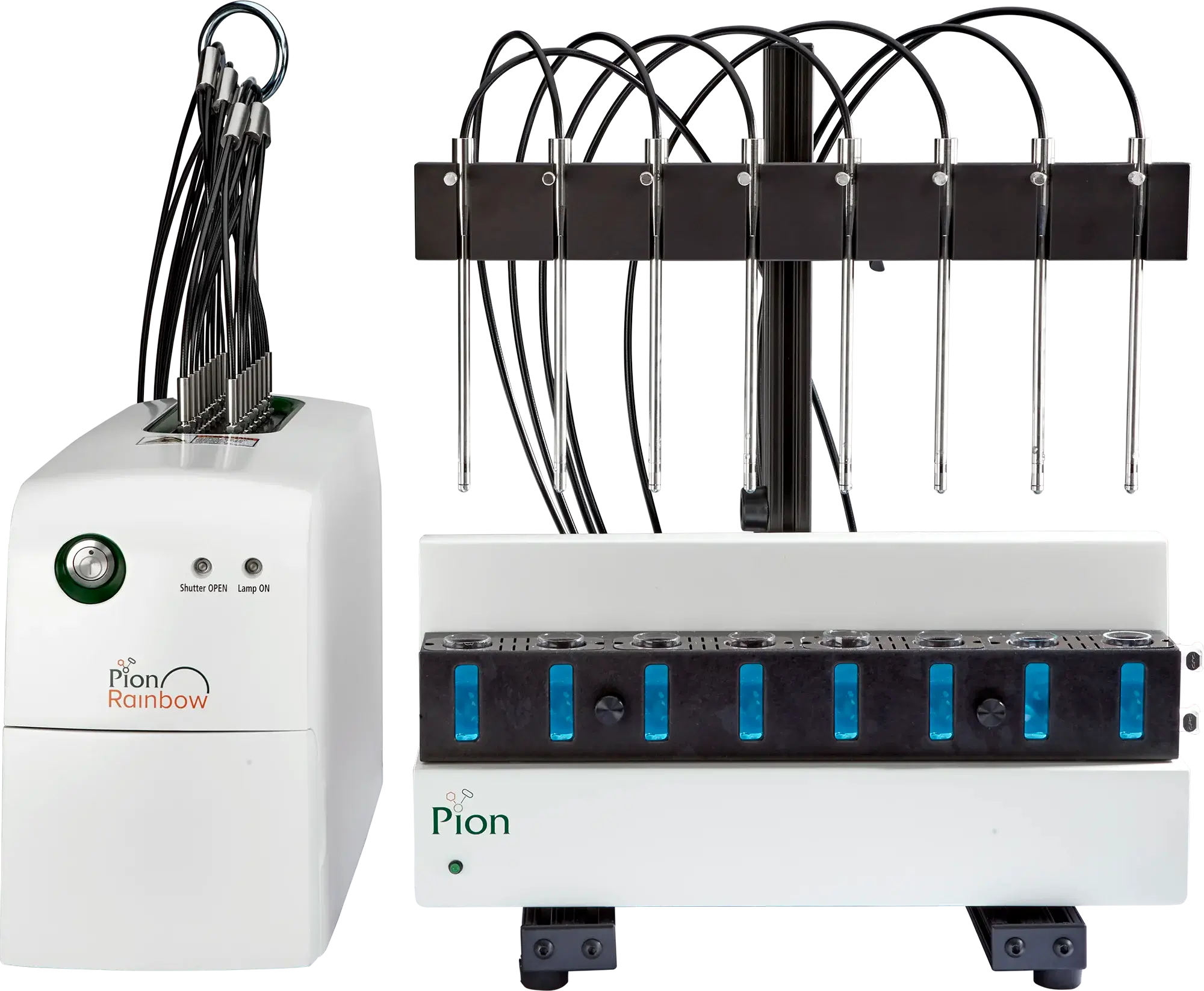
PAMPA Kit
Parallel artificial membrane permeability assay







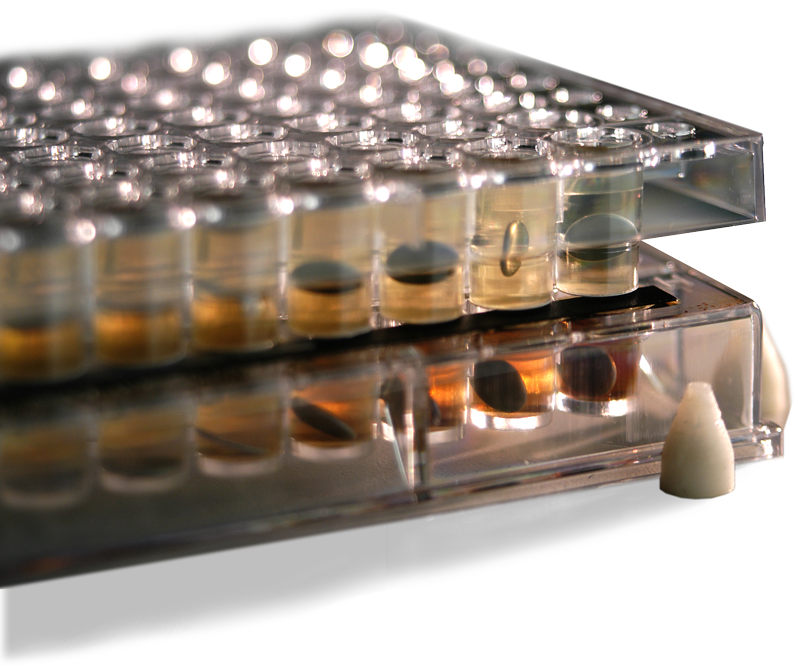

Pion’s PAMPA is an in vitro, high throughput screening assay highly correlated to biological membrane permeability

Ideal for screening excipients, solvents, and APIs to rank order formulations and drug candidates on the basis of permeability.

Each PAMPA well uses artificial lipid membranes that mimic drug uptake in the gastrointestinal tract (GIT) or the blood-brain barrier (BBB) to provide robust, reliable, biorelevant results.
Double-Sink Method
PAMPA enables efficient evaluation of the passive permeability of APIs and the absorption potential of simple or complex formulations. By effectively mimicking many of the properties of biological membranes it can minimize reliance on expensive in vivo/in situ studies, reducing the time and cost for selecting drug candidates. Pion’s Double-Sink™ PAMPA assay also allows formulators to simultaneously study how excipients impact the solubility and permeability of APIs, to accelerate formulation screening activities



The Pion PAMPA Advantage
Complete Solution
The complete PAMPA system includes our 96 well STIRWELL plates, the Gut-Box™ stirring system and PAMPA Explorer™ software.
High Throughput
The STIRWELL plates in Pions’ PAMPA sandwich kits contain a pre-loaded stir bar in each well, providing dynamic stirring for each assay, allowing for high-throughput screening of multiple compounds simultaneously.
Reproducible Results
While orbital shakers can lead to edge effects in the assay plates, GutBox provides consistent agitation across the whole plate, allowing for more reproducible data.
In Vivo Correlation
Gut-Box controls stirring speed to match the hydrodynamics of the intestinal tract or the blood brain barrier. The adjustable stirring allows you to mimic the ABL (aqueous boundary layer), allowing for higher accuracy and reducing assay time.
Easy Data Interpretation
PAMPA Explorer software for easy data interpretation. SW includes a plate-reader data wizard, results visualization, and reporting. Data output is the complete mass balance including membrane retention.
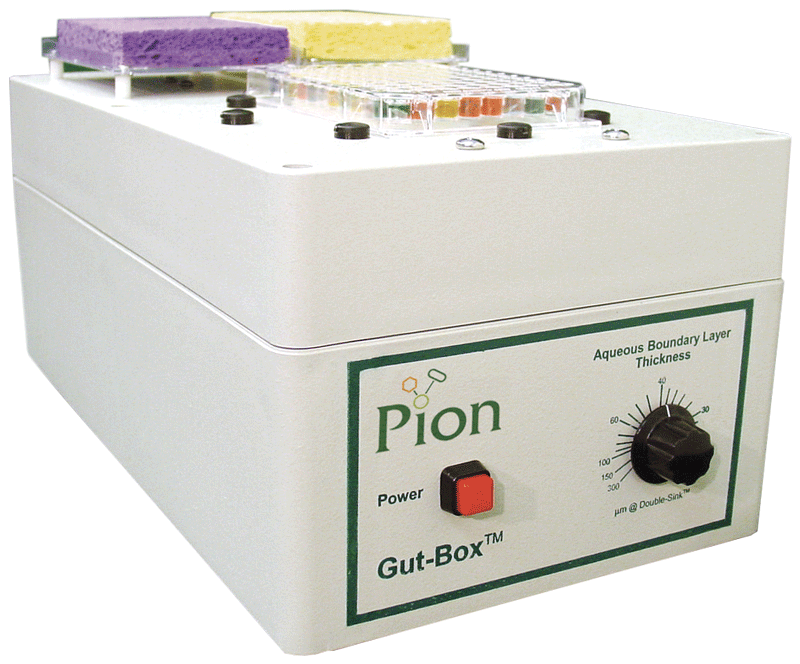
The Gut-Box
The Gut-Box was designed by Pion to complement our PAMPA Systems, resulting in the most comprehensive turn-key assays in the market for predicting in vivo permeability. Mount any 96 well plate (loaded with stirrers) on top of the Gut-Box and choose your stirring speed. The Gut- Box stirring mimics the hydrodynamics for various bio-mimetic models. It extends the permeability response for an additional order of magnitude, allowing more accurate biological membrane permeability predictions.