MacroFLUX
In-vitro assessment of dissolution-absorption processes from full dosage forms.







In-vitro assessment of dissolution-absorption processes from full dosage forms.
Combine Dissolution and Absorption measurements into a single assay.
A stirred absorption chamber into familiar dissolution apparatus
Extend the utility of in situ UV concentration monitoring to provide an accurate assessment of transmembrane FLUX from whole dosage forms
Real-time dissolution and absorption concentration monitoring
The filter-supported artificial permeation membrane separates the dissolution (donor) compartment from the receiver compartment, containing pH 7.4 Pion Acceptor Sink Buffer.
The dissolution compartment typically holds 900 mL of compendial or biorelevant dissolution buffer. The modified vessel cover allows fiber-optic UV probes to be positioned in both the donor and receiver compartments allowing real-time dissolution and absorption concentration monitoring in both chambers. Concentration monitoring is enabled by connecting the fiber optic UV probes from the Rainbow Dynamic Dissolution Monitor® instrument.
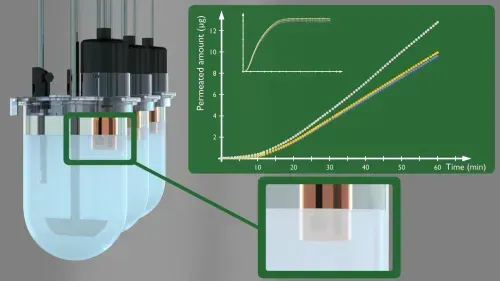



MacroFLUX can be used:
- To conduct simultaneous dissolution-absorption studies
- During formulation development for optimizing product performance and to evaluate whether certain formulation changes will affect the bio-performance of a certain drug product
- During life-cycle management and product extensions to demonstrate bioequivalence
- To compare brand and generic formulations to demonstrate bioequivalence
- To compare test to reference formulations
- To ensure continuity of product quality (batch to batch consistency) and performance of the manufacturing process (in order to differentiate bioequivalent batches from non-bioequivalent batches within a range that guarantees comparable biopharmaceutical performance in vivo).








