SiriusT3
Automated platform for the determination of Physicochemical Properties







Automated platform for the determination of Physicochemical Properties
Elucidate drug potential
Globally renowned as the gold standard technique for determination of pKa, logP/D and solubility of ionisable compounds.
Rich insights to discovery scientists allowing them to make accurate and assured decisions about their drug candidates.
Determine pKa values using two techniques: spectrometric (for UV-active compounds) and potentiometric (for non-UV active compounds). With high throughput automated screening capabilities, SiriusT3 can complete up to 80 assays a day.
Determine the intrinsic solubility of the different forms (crystalline and amorphous) for polymorphs compounds using the Pion’s CheqSol technique.
Sirius T3 Benefits
SiriusT3 is the only fully automated instrument designed for compound screening and lead optimization by investigating the physicochemical properties of a sample in vitro.
- Requiring only sub-milligram sample quantities in most cases, the SiriusT3 can complete the analysis in less than 6 minutes per titration using the high throughput pKa screening**.**
- Determine pKa values using two techniques: spectrometric (for UV-active compounds) and potentiometric (for non-UV active compounds).With high throughput automated screening capabilities, SiriusT3 can complete up to 80 assays a day.
- Measure log P/D as a function of pH in less than 2 hours using the potentiometric technique in place of traditional shake-flask methods. Determine kinetic and intrinsic solubility using Pion’s patented CheqSol technique within two hours.
- Use the CheqSol technique to provide intrinsic solubility as a function of pH and generate detailed information about the extent and duration of the supersaturated states.


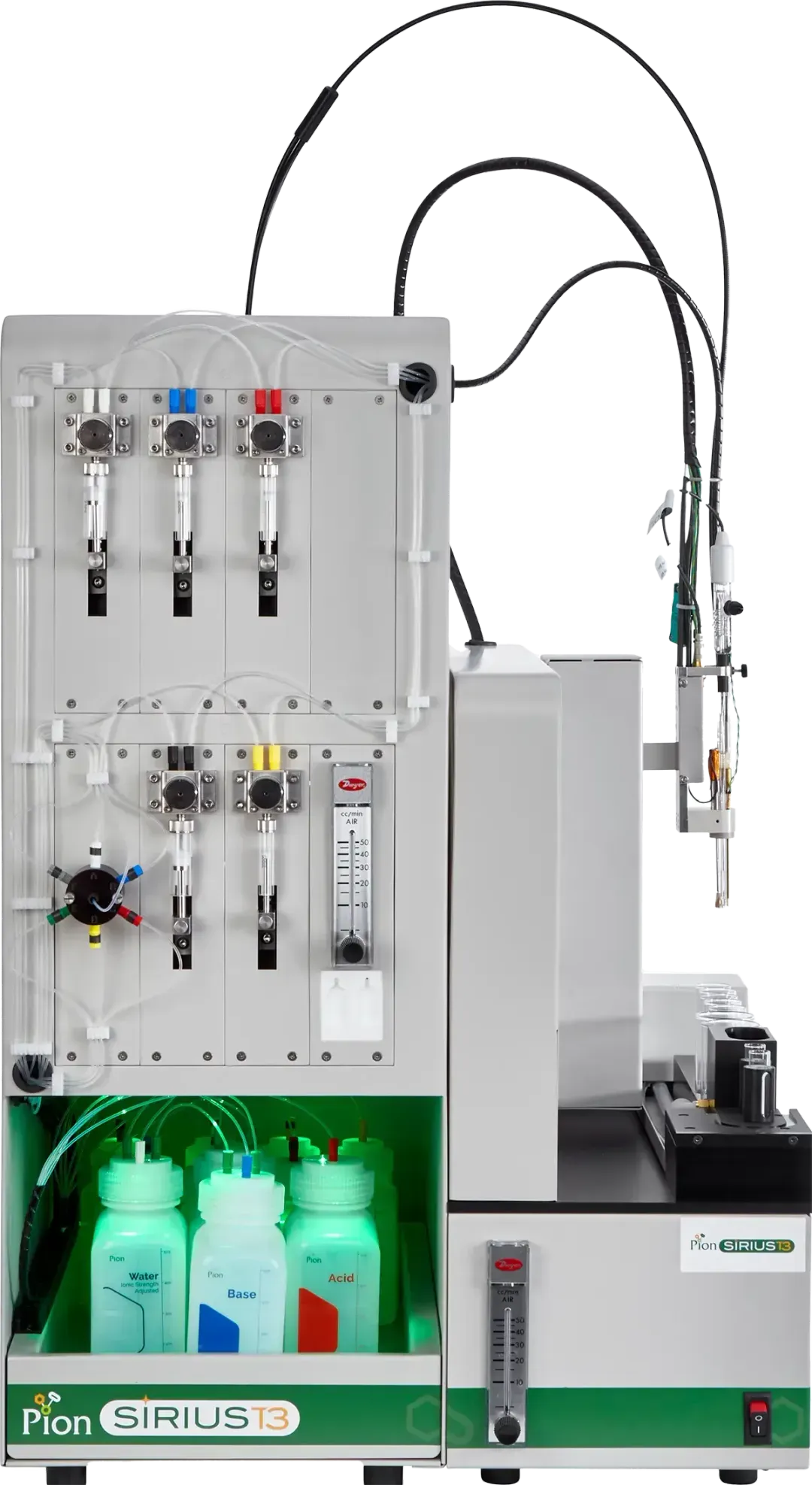
Use the SiriusT3 in the format that works in your lab by choosing one of four different configurations
DTAu –Fully automated with pKa high throughput screening SiriusT3 system with both spectroscopic and potentiometric assay capabilities.
DTu – A single vial system for one assay at a time with both spectroscopic and potentiometric assay capabilities.
DTA – A fully automated system with only potentiometric assay capabilities.
Lite - A single vial system for one assay at a time with only potentiometric assay capabilities.
Assay Expert – Optional integrated prediction module
SiriusT3 Control and SiriusT3 Refinement
A powerful analytical software designed for use with the instrument which collects UV spectra at each pH for automated data analysis.


Assay Expert – Optional integrated prediction module
Assay Expert applies the predictive software of ACD/Labs to automatically optimize the design of the experiments.





