SiriusT3
Fully automated titrator UV/VIS platform for drug characterization and compound screening







Highly accurate pKₐ, Log P/D and solubility measurement
The SiriusT3™ is a fully automated solution for drug characterization. Integrating seamlessly with analytical workflows in drug discovery, it helps researchers to identify the most promising drug candidates rapidly and efficiently, reducing the risk of late-stage failures. An integral tool for compound screening, the SiriusT3 enables highly accurate pKₐ, Log P/D and solubility measurements in a single instrument using sub-mg of sample.

Comprehensive Data, Confident Decisions
With online monitoring, the SirusT3 provides detailed titration curves and UV/VIS spectra collected at every single pH to help researchers to make quick decisions
pKₐ Perfection
High throughput pKₐ screening; fully automated assays producing results in ~6 mins per titration. Validated pKₐ data by applying both spectroscopic and potentiometric techniques.
Advanced Automation
Generating detailed information about the extent and duration of compounds’ supersaturated states, intrinsic and kinetic solubilities, and pH-solubility within a single automated assay in less than two hours.
Highly accurate pKₐ, Log P/D and solubility measurement
SiriusT3 Benefits
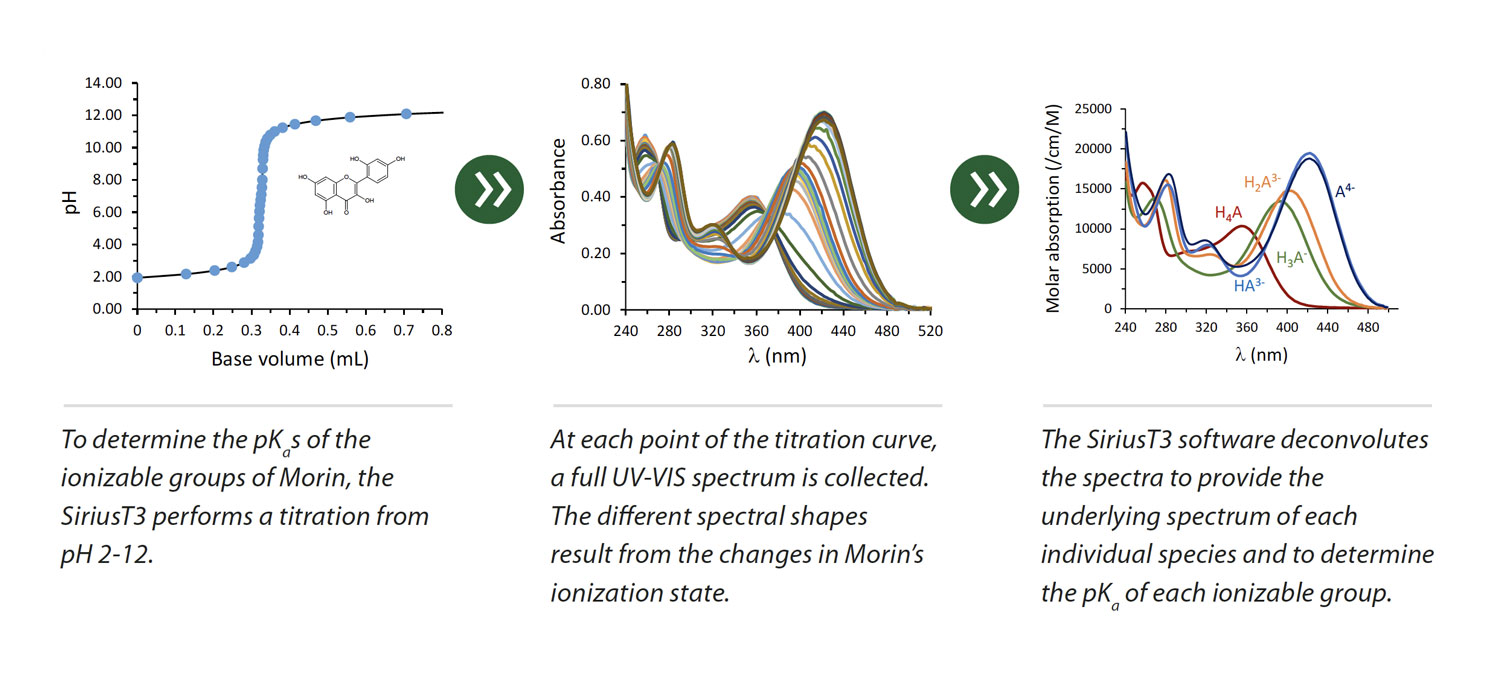
Determine pKₐ values using two techniques: spectrometric (for UV-active compounds) (Fig. 1) and potentiometric (for both UV and non-UV active compounds). With high throughput automated screening capabilities, SiriusT3 can complete up to 80 assays a day.
Unlike traditional shake flask methods, Pion’s SiriusT3 provides comprehensive and detailed information about the behavior of pharmaceutical drugs from titration curves, UV data, and time dependent solubility.
(Fig. 1) Pion’s unique, proprietary Cheqsol method enhances instrument capabilities enabling the accurate determination of kinetic and intrinsic solubilities for ionizable compounds, characterization of supersaturation behavior and the generation of pH-solubility profiles. The SiriusT3 is the only commercially available fully automated system combining in vitro physicochemical property measurements with high-throughput screening capabilities.


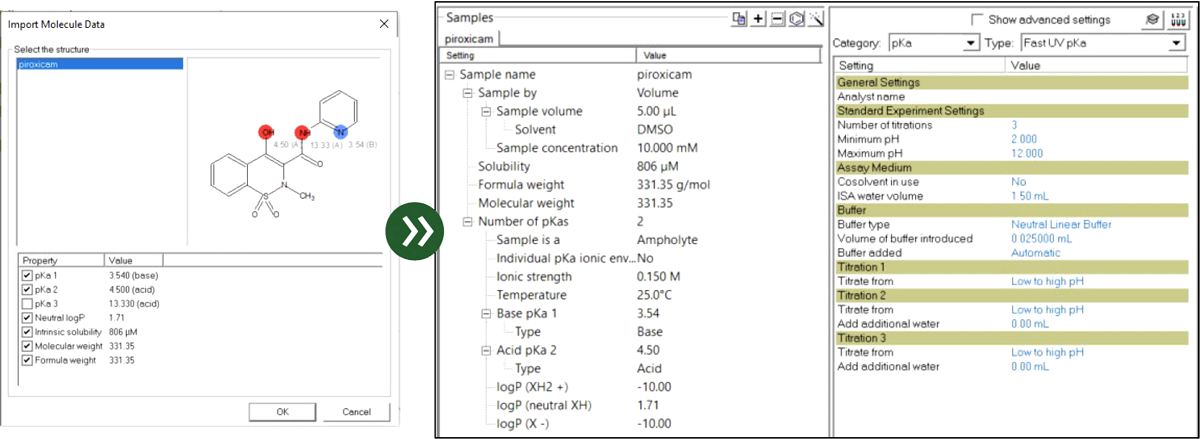
SiriusT3 Software
Our software integrates SiriusT3 Control which allows the full automation of the system, and SiriusT3 Refine for the data processing, applying complex computational algorithms to produce high integrity data.
An optional upgrade of the software is Assay Expert, an integrated prediction module for structural analysis and the identification of ionizable groups. Determine acidity/basicity and estimate pKₐ and log P values. Use the resulting data to optimize experimental design.